- Lab Professionals
- Trends & Topics
Main laboratory abnormalities associated with COVID-19
Although RT-PCR is the recommended testing for diagnosis of a SARS-CoV-2 infection, there are numerous other diagnostic parameters that show abnormal laboratory results in COVID-19 patients.
In addition to hematologic parameters, like lymphocyte counts, especially CRP testing and a series of other clinical chemistry parameters, as well as D-Dimer testing play a role in monitoring and staging of COVID-19 patients.1, 2
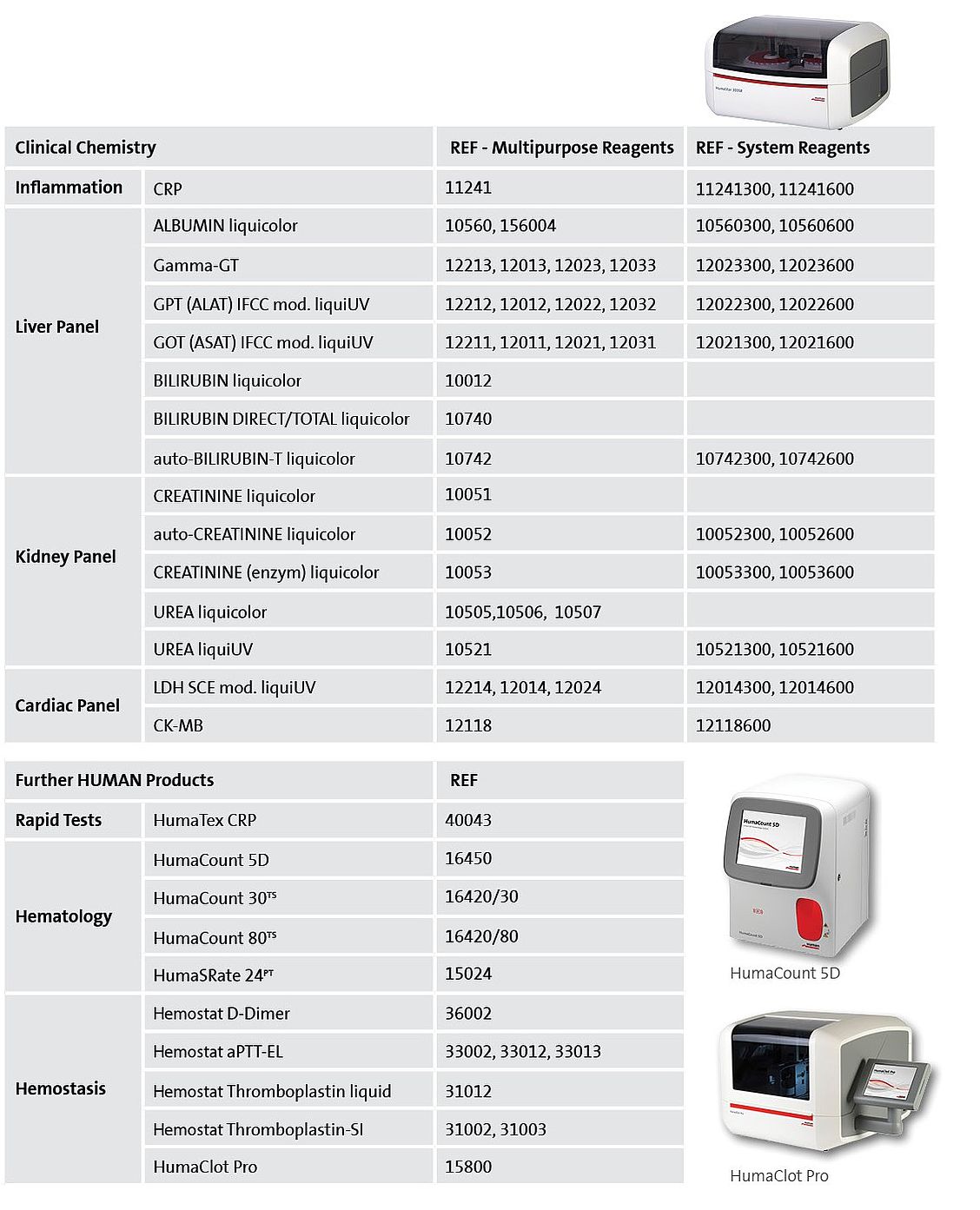
Suitable HUMAN products in the context of COVID-19
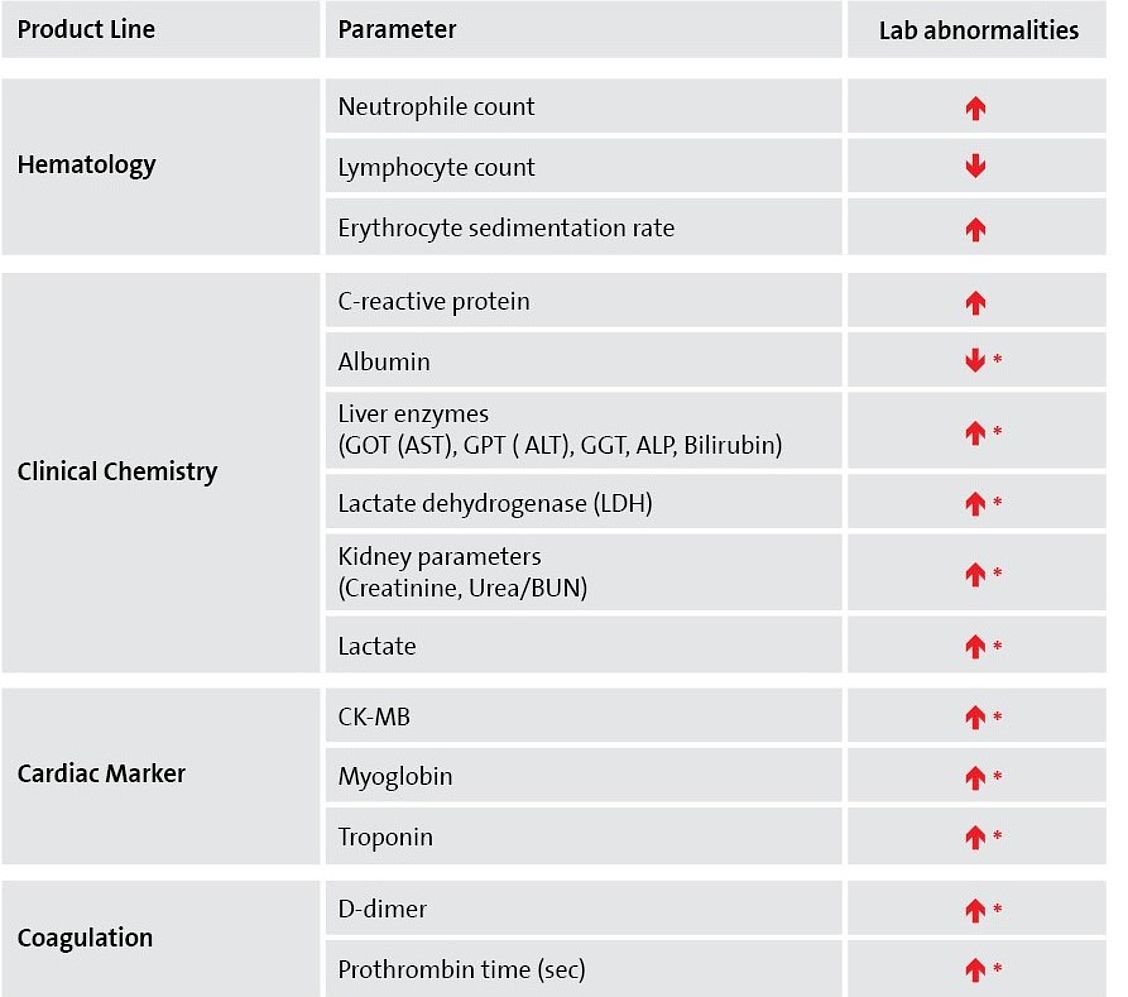
* in severe cases, mainly
1 Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7);
Released by National Health Commission & State Administration of Traditional Chinese Medicine; March 3, 2020
2 Lippi G, Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med 2020 Feb 24. doi: 10.1515/cclm-2020-0198