- Lab Professionals
- Trends & Topics
Malaria testing solutions at a glance
The spread of vector-borne diseases is a public health threat. Availability of appropriate diagnostic solution for disease management and eradication is still a challenge.
Out of the five Plasmodium parasite species, four (P. falciparum, P. vivax, P. ovale, and P. malariae) are human malaria species. In humans the parasites multiply exponentially in the liver and after several developmental stages in infected red blood cells (RBCs).
Although P. falciparum and P. vivax are the most important species due to their widespread prevalence, malaria caused by P. falciparum is the deadliest form1. The P. falciparum parasite is able to avoid the body's immune system for most of its life cycle. It replicates rapidly in the blood so the infection can quickly progress to a point where the disease becomes very severe. Additionally, falciparum causes the surface characteristics of the infected RBCs to change making them "sticky." These RBCs stick to the endothelial cells that line the lumen of blood vessels.
These RBCs stick to the endothelial cells that line the lumen of blood vessels. This adhesive property of RBCs poses the greatest risk of vascular occlusions - especially in the capillaries, causing cerebral malaria, or in the kidneys - resulting in hypoxia and serious organ damage.
Rapid and accurate diagnosis of new and/or asymptomatic diseases is critical for effective treatment, control and elimination. Laboratory tests including nucleic acid amplification techniques (NAATs), rapid diagnostic tests (RDTs), ELISA and traditional microscopic examination of blood smears are some of the available diagnostic platforms.
We at HUMAN currently offer a Malaria Rapid Test and in the field of molecular diagnostics the Malaria-LAMP solution.
Nucleic acid amplification techniques (NAATs) such as real-time PCR are capable of identifying subclinical, submicroscopic infections. Still, real-time PCR remains inaccessible in resource-limited and remote settings due to the expensive infrastructure, reagents and technical expertise required. Isothermal molecular diagnostic techniques such as loop mediated isothermal amplification (LAMP), nucleic acid sequence based amplification (NASBA), thermophilic helicase dependent amplification (tHDA) and recombinase polymerase amplification (RPA) have the potential to combine high analytical sensitivity with technical requirements that facilitate application even in remote settings.
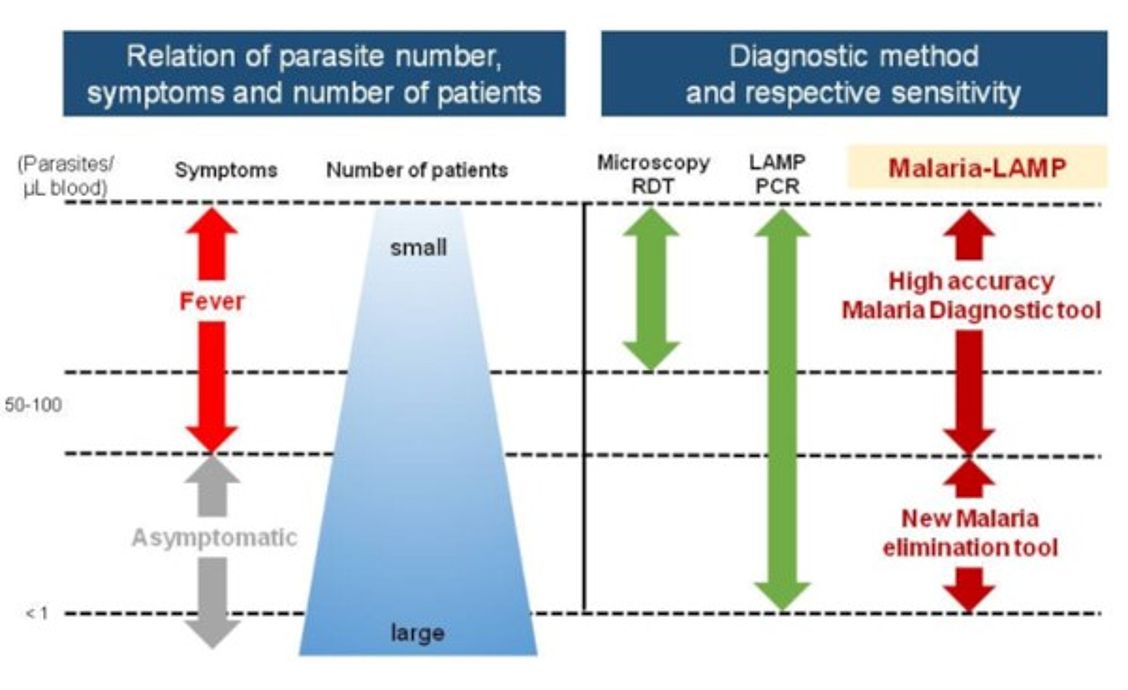
Schematic overview: Distribution of Malaria infections and the possible role of
Malaria-LAMP; source: Eiken Chemical Co, LTD, April 2018
Now, according to FIND, LAMP has become a forerunner in this respect as it offers the benefits of sensitive and specific testing combined with minimal set up requirements and high throughput capabilities2,3.
Many regions of the world where malaria is endemic suffer from poor access to laboratory-based diagnostics. In community settings and health facilities without laboratories, rapid diagnostic tests (RDT) for the direct detection of antigens of the Plasmodium species provide the best possible means to diagnose acute malaria. The WHO has acknowledged the unique value of RDTs for malaria by including them in its List of essential diagnostics as of its 1st edition in 2019.
Lateral flow immunochromatographic assay (LFA, also known as strip test) is a type of RDT that features a particularly favourable combination of performance, stability, ease of use, robustness and cost. HUMAN supports WHO’s global strategy for malaria aiming to reduce global mortality rates and case incidence by providing a reliable and affordable RDT – the Hexagon Malaria LFA.
Hexagon Malaria is a rapid qualitative immunochromatographic LFA to help diagnose acute malaria in human whole blood. The test is sensitive to all Plasmodium species that commonly infect humans and allows to differentiate between malaria disease induced by P. falciparum or one of the other Plasmodium species (P. vivax, P. malariae, P. ovale). The differentiation is made possible by two complementary test lines on the strip membrane. Antibodies deposited on one of the test lines capture complexes of dye with the HRPII antigen which is unique to the P. falciparum species. This line can therefore only be resolved in cases of the potentially life-threating falciparum malaria. The other test line harbours antibodies that capture complexes of dye with the less specific pLDH antigen shared by all Plasmodium species that are pathogenic to humans. This line becomes visible in cases of malaria disease independent of the specific Plasmodium species that has infected the patient.
Malaria-LAMP
Simple and fast Malaria-LAMP workflow
The LoopampTM workflow allows for the easy DNA extraction and preparation of reaction mixes in a few steps without any additional equipment. Reagent storage and shipment at room temperature and the excellent test performance make molecular pathogen testing available in rural areas with limited settings.
- High specificity and sensitivity
- Very short turnaround times
- Simple processing
- High degree of robustness
- Easy result interpretation, either visually (HumaLoop) or by means of a turbidimeter (HumaTurb system)
High reliability ensured by best performance
- Sensitivity: 96 – 100 %
- Specificity: 97 – 100 %
- Detection limit: 1 parasite / μl
- Differentiation between Plasmodium pan species, P. falciparum and P. vivax
- Listed in the WHO Policy brief on malaria diagnostic in low-transmission settings
- Regulatory status: CE IVD
HUMAN Hexagon Malaria (REF 58054) – key parameters
- Test format: standardised kit containing 25 LFA devices,
sample diluent in a dropper bottle, capillary tubes and the instructions for use - Sample type: whole blood (capillary from fingerstick or venous)
- Diagnostic sensitivity : Pf 99.5%, non-Pf 96.0%
- Diagnostic specificity: 99.6%
- Time to result: 10 minutes
- Storage: 1…40 ° C
- Power requirements: none
- Need for calibration/spare parts/equipment to read result: none
- Regulatory status: CE IVD
References
- World Health Organization Website, WHO Global Malaria Programme: World Malaria Report: 2020.
- Vaughan, A.M., A.S.I. Aly, and S.H.I. Kappe, Malaria Parasite Pre-Erythrocytic Stage Infection: Gliding and Hiding. Cell Host & Microbe. 4(3): p. 209-218.
- Zimmerman, P.A., et al., Red blood cell polymorphism and susceptibility to Plasmodium vivax. Adv Parasitol, 2013. 81: p. 27-76.