- Lab Professionals
- Trends & Topics
Malaria: Small Bites, Global Threat, Diagnostic Challenge
Public health threatened by the spread of vector-borne diseases. Availability of appropriate diagnostic solution for disease management and disease elimination is still a challenge. A new approach might change the situation for the fight against malaria.
There has been a renewed interest in VBDs due to the recent spread of Zika virus, which is linked to causing microcephaly birth defect.
VBDs are often fatal and/or debilitating. They constitute more than 17% of global infectious diseases. Every year more than 700,000 people die from vector borne diseases1. Although mostly found in the tropical and sub-tropical regions, increasing global travel, urbanization, and climate change contribute to VBD outbreaks in new regions. Not only in resource-limited settings, there is still a lack of rapid, accurate and cost effective diagnostic testing. This makes VBDs among the most difficult of all infectious diseases to prevent and control. Therefore, a major way of disease prevention is to make people aware of accurate diagnosis, sanitation habits and vector control mechanisms. HUMAN is committed fighting infectious diseases, especially in resource-limited settings.
Examples of VBDs with the respective vector and pathogen
| Disease | Vector | Pathogen |
|---|---|---|
| Malaria | Female mosquito (Anopheles genus) | Plasmodium parasite |
| Dengue | Female mosquito (Aedes aegypti) | Dengue virus |
| Zika | Aedes species mosquito | Zika virus |
| Chagas | Triatomine bug | Trypanosoma cruzi |
| African Sleeping Sickness | Tsetste fly | Trypanosoma brucei |
| Leishmaniasis | Female sandflies | Leishmania parasite |
| Schistosomiasis | Freshwater snail | Flatworm (Schistosoma genus) |
| West Nile | Mosquito | West Nile virus |
| Japanese encephalitis | Mosquito (Culex species) | Japanese encephalitis virus |
| Yellow fever | Mosquito (Aedes and Haemogogus species) | Yellow fever virus |
Malaria - Diagnostics at the Forefront for Disease Management and Elimination
Rapid and accurate diagnosis of new and/or asymptomatic diseases is critical for effective treatment, control and elimination of VBDs. Cutting-edge laboratory tests including nucleic acid amplification techniques (NAATs), rapid diagnostic tests (RDTs), ELISA and traditional microscopic examination of blood smears are some of the available diagnostic platforms.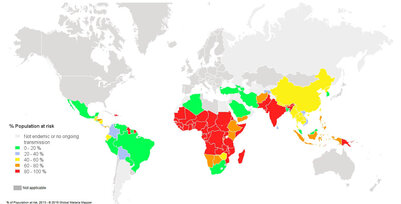
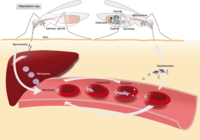
Out of the five Plasmodium parasite species, four (P. falciparum, P. vivax, P. ovale, and P. malariae) are human malaria species. In humans the parasites multiply exponentially in the liver and after several developmental stages in infected red blood cells (RBCs).
Although P. falciparum and P. vivax are the most important species due to their widespread prevalence, malaria caused by P. falciparum is the most deadly form2. The P. falciparum parasite is able to avoid the body's immune system for most of its life cycle. It replicates rapidly in the blood so the infection can quickly progress to a point where the disease becomes very severe. Additionally, . falciparum causes the surface characteristics of the infected RBCs to change making them "sticky." These RBCs stick to the endothelial cells that line the lumen of blood vessels. This adhesive property of RBCs poses the greatest risk of vascular occlusions - especially in the capillaries, causing cerebral malaria, or in the kidneys - resulting in hypoxia and serious organ damage.
Fig. 1. Population at risk contracting malaria according to data from 2013 3
Quick Facts and Figures
- Risk: An estimated 3.4 billion people worldwide are at a risk (Fig. 1)
- Morbidity and mortality: 216 million clinical cases and 445,000 deaths in 2016
- 2016 mortality: 91% of deaths in Africa alone
Challenges in Malaria Diagnostics
Accurate and rapid diagnosis is critical for effective malaria treatment, control and elimination. Unselective malaria treatment for suspected diseases paves the way for the rising levels of anti-malarial drug resistance (FIND). Currently, WHO recommends prompt malaria diagnosis in all patients with suspected malaria either by microscopy or malaria rapid diagnostic test (RDT).
A major drawback of microscopy is the need for well-trained, competent microscope specialists. This is impractical especially in field settings. Rapid diagnostic tests (RDTs) are ideal especially in field settings. However, only a few of the available RDTs can directly detect P. vivax infections and none can directly detect the dormant reservoirs of the vivax parasite in the liver, see below.
Additionally, subclinical infections in remote settings cannot be diagnosed.
Related Products
As sensitive as PCR, as robust as RDT
Nucleic acid amplification techniques (NAATs) such as real-time PCR are capable of identifying subclinical, submicroscopic infections. Still, real-time PCR remains inaccessible in resource-limited and remote settings due to the expensive infrastructure, reagents and technical expertise required. Isothermal molecular diagnostic techniques such as loop mediated isothermal amplification (LAMP), nucleic acid sequence based amplification (NASBA), thermophilic helicase dependent amplification (tHDA) and recombinase polymerase amplification (RPA) have the potential to combine high analytical sensitivity with technical requirements that facilitate application even in remote settings. Now, according to FIND, LAMP has become a forerunner in this respect as it offers the benefits of sensitive and specific testing combined with minimal set up requirements and high throughput capabilities5,6.
LAMP opens the door for effective malaria elimination campaigns and projects. In addition to P. falciparum, specific detection of Malaria vivax will also be possible with LAMP in the near future (assay is under development).
In conclusion, the control and elimination of malaria reliable detection of subclinical cases is indispensable. The good news is that appropriate technologies like Malaria-LAMP are available.
Malaria-LAMP is a new tool that detects asymptomatic carriers to achieve Malaria elimination
Important considerations for malaria diagnostics
- Malaria relapses
P. vivax has a unique feature in its life-cycle. After invading human hepatocytes, it can either continue with its course of infection or persist for a long time as dormant hypnozoites. After a subsequent activation, which can be after some months or several years, the hypnozoites cause clinical symptoms in the absence of a new mosquito bite2. The hypnozoites are hard to detect because the parasite wraps itself in the cell membrane of the infected host liver cell and is relatively protected from an attack from the body’s immune system6. Currently there is a lack of bio-markers and diagnostic techniques to detect the dormant hypnozoites. The only way to diagnose patients infected by the dormant liver stage is after activation of the hypnozoites and the ensuing relapse. - Resistance to P. vivax in certain populations
P. vivax has a wider distribution than P. falciparum because it is able to develop in the Anopheles mosquito vector at lower temperatures, and to survive at higher altitudes and in cooler climates. Additionally, it can form dormant hypnozoites. P. vivax uses the Duffy antigen, present on erythrocytes, as receptors to attach and invade them. Therefore, individuals with erythrocytes lacking the Duffy antigen, as seen among many African populations, are resistant to infection7. However, there have been reports of presence of P. vivax in Duffy negative individuals suggesting that erythrocyte receptors other than Duffy antigen can be used to invade erythrocytes8. Such findings reveal the potentially enormous impact in P. vivax distribution and considered while diagnosing malaria. - Antimalarial drug- resistance
There is an alarming emergence of anti-malarial drug resistant P. falciparum in countries across Asia and Africa. This is threatening the gains made in reducing the morbidity and mortality from malaria9. Diagnostic testing for anti-malarial drug resistance should be considered before starting treatment.
References
-
Vector-borne diseases. (accessed on 24.04.2018): http://www.who.int/en/news-room/fact-sheets/detail/vector-borne-diseases
-
World Health Organization Website, WHO Global Malaria Programme: World Malaria Report: 2017.
-
Vallejo, A.F., et al., Evaluation of the loop mediated isothermal DNA amplification (LAMP) kit for malaria diagnosis in P. vivax endemic settings of Colombia. PLoS Negl Trop Dis, 2015. 9.
-
Hopkins, H., et al., Highly sensitive detection of malaria parasitemia in a malaria-endemic setting: performance of a new loop-mediated isothermal amplification kit in a remote clinic in Uganda. J Infect Dis, 2013. 208.
-
Vaughan, A.M., A.S.I. Aly, and S.H.I. Kappe, Malaria Parasite Pre-Erythrocytic Stage Infection: Gliding and Hiding. Cell Host & Microbe. 4(3): p. 209-218.
-
Zimmerman, P.A., et al., Red blood cell polymorphism and susceptibility to Plasmodium vivax. Adv Parasitol, 2013. 81: p. 27-76.
-
Mendes, C., et al., Duffy negative antigen is no longer a barrier to Plasmodium vivax--molecular evidences from the African West Coast (Angola and Equatorial Guinea). PLoS Negl Trop Dis, 2011. 5(6): p. e1192.
-
Ashley, E.A., et al., Spread of Artemisinin Resistance in Plasmodium falciparum Malaria. N Engl J Med, 2014. 371(5): p. 411-423.
-
Britton, S., Q. Cheng, and J.S. McCarthy, Novel molecular diagnostic tools for malaria elimination: a review of options from the point of view of high-throughput and applicability in resource limited settings. Malar J, 2016. 15: p. 88.